10 Basics of Endovascular Surgery (Endo Basics)
The pre/post questions are listed below. They are all multiple choice questions with a single right answer. To best guide your learning, we have hidden the answers in a collapsible menu. Before reading the chapter, we suggest giving the questions a try, noting your answers on a notepad. After reading the chapter, return to the questions, re-evaluate your answers, and then open the collapsible menu to read the correct answer and discussion. Do not fret if you have difficulty answering the questions before reading the chapter! By the end of the chapter, we are certain you will have covered the knowledge necessary to answer the questions. There will be a teaching case at the end of the chapter. This is another opportunity to exercise your new knowledge!
Pre/Post Questions
Case Based Questions
- A 77-year-old female with a past medical history of CAD, CHF with an EF of 14%, COPD, and a prior total thyroidectomy comes in for evaluation of carotid stenosis. She had a TIA with a right-sided facial droop two weeks ago and was briefly admitted to this hospital. She had a carotid US and was found to have an 85% left carotid stenosis. Which of the following treatment modalities would be best suited for the patient?
Open carotid endarterectomy
Transcarotid Artery Revascularization (TCAR)
No intervention because of her age
No intervention because of her medical comorbidities
- A 66-year-old male presents to the emergency room one week after a percutaneous coronary intervention via right groin access. He reports that over the last few days, he has noticed a bulge in his groin and when he touches it, it is painful and has a pulsatile nature. A complete exam reveals a pulsatile, tender mass in the right groin with no skin changes, but with a bruit on auscultation. Which of the following complications is NOT associated with this patient’s diagnosis?
Embolization to the lower extremity
Rupture of the mass
True femoral artery aneurysm
Compression of the femoral vein
Gangrene of the toes
- A 42-year-old patient with a history of an arterial thrombus four years ago presents to the ER with severe right lower extremity pain. A CTA is obtained showing no atherosclerotic disease but with acute thrombus along the distal SFA and popliteal artery. You take the patient to the operating room for a lower extremity angiogram. Which catheter would be most useful to help break up the clot over time?
Penumbra thrombectomy catheter
Cragg Mcnamara Catheter
Fogarty Catheter
Peripherally inserted central venous catheter
- A 70-year-old male is brought to the operating room for an EVAR procedure for his 6 cm infrarenal AAA. You have chosen a graft to use and the nurse is asking if there are any wires that you would like opened to deliver the device into the patient’s aorta. Which of the following wires would be most useful for this task?
0.018” flexible Terumo wire
0.035” Amplatz wire
0.035” floppy Glidewire
0.014” Pilot wire
- A 63-year-old smoker presents to the clinic with left lower extremity claudication and an ischemic ulcer. He is taken to the operating room for an angiogram via right groin access. Which of the following steps is INCORRECT when obtaining arterial access?
Using anatomic landmarks to confirm cannulation through the inguinal ligament
Using ultrasound to ensure cannulation of the common femoral artery
Using fluoroscopy to ensure puncture at the level of the femoral head
Using the Seldinger technique for first access, making sure not to lose access to the artery during exchanges or wires and catheters.
Introduction
Endovascular surgery is a minimally invasive approach to treat a variety of vascular pathologies including peripheral artery disease, aortic aneurysms, venous diseases, and more. Endovascular surgery is not limited to vascular surgery as a specialty and is also employed by interventional cardiologists, neurosurgeons, interventional radiologists, and others.
Over the past 30 years, endovascular surgery has dramatically changed the practice of vascular surgeons, decreasing patient morbidity and mortality in the immediate postoperative period and the length of hospital stay.
Understanding endovascular surgery as a medical student or junior resident can be a difficult task due to the abundance of wires, catheters, sheaths, and devices used and orienting onself using fluorscopic images. In this chapter, we will focus on developing a conceptual basis for endovascular surgery.
Relevant Anatomy and Vascular Access
The common femoral artery (CFA) is the most common site of access for endovascular surgery due to its larger size and lower risk of complications relative to other sites of vascular access. Other sites of arterial access include the radial, brachial, and axillary artery in the upper extremity, as well as the popliteal and dorsal pedal arteries in the leg. The further away the vessel is from the aorta, the smaller the vessel lumen, and hence the smaller the diameter of devices that can be utilized in that artery.
The CFA is referred to as the external iliac artery proximal to the inguinal ligament and the superficial femoral artery (SFA) distal to the branching of the profunda femoris artery (PFA). The anatomic relationship of the CFA to the surrounding vessels can be remembered with the mnemonic “NAVeL”, femoral nerve, artery, vein, and lymph nodes from lateral to medial. The Femoral Triangle consists of these three vessels and nerves surrounded by the inguinal ligament superiorly, sartorius muscle laterally, and the adductor longus medially.
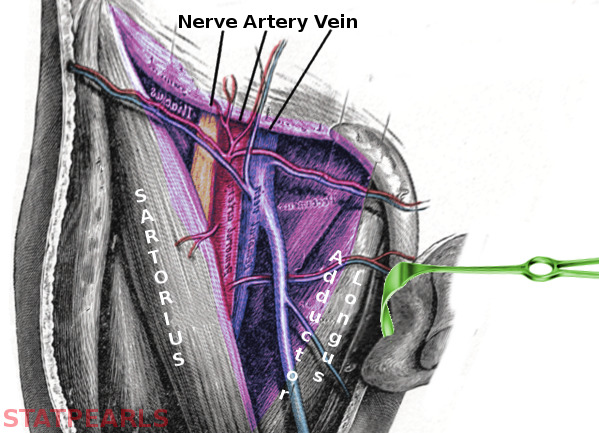
Correct anatomic positioning for cannulation of the CFA can be achieved by palpating the point of maximum pulsation inferior to the inguinal ligament, a structure identifiable by tracing along the facial plane between the anterior superior iliac spine and pubic symphysis. The following imaging modalities assist with anatomic positioning:
Fluoroscopic guidance: cannulation of the CFA should be attempted at the level of the middle to inferior femoral head.
Ultrasound (US) guidance: identification of the PFA ensures cannulation is attempted of the CFA in place of the SFA. At the level where the great saphenous vein joins the femoral vein, US will show one large vessel surrounded by two smaller vessels, which can be remembered as the “Mickey Mouse Sign.”
An in depth review of lower limb anatomy can be found in the APDVS Claudication Chapter or here.
Ultrasound Guided Access
There is emerging evidence to suggest real time identification of anatomic structures with US guidance may decrease the risk of access-related complications. A summary to the steps of US-guided access of the CFA are included below.
Identifying the correct vessel:
- Obtain baseline peripheral pulses.
- Obtain a micropuncture kit, containing a hollow needle, guidewire, and catheter
- Using the US probe, identify any plaque or calcification along the CFA or iliac artery that may change the site of access.
- Identify the CFA, which can be differentiated from the femoral vein by its thick wall, resistance to compression, pulsatility, and relative lateral anatomic positioning. A video identifying the CFA through the “Mickey Mouse Sign” on US is available here.
Obtaining access through the Seldinger technique:
The Seldinger technique help obtain safe access to blood vessels. In contrast to previous methods, the Seldinger technique causes minimal damage to the blood vessel thereby allowing the puncture to be closed percutaneously, and avoids the risk of loss of access during the procedure through keeping at least one wire, catheter, or sheath at the site of interest in the body all times.
Steps to the Seldinger technique are as follows:
- Cannulate the CFA by inserting the hollow needle at a 45-90 degree angle to the skin under US guidance, blood will flow out of the needle once inserted into the vessel.
- Insert the guidewire into the vessel. Remove the hollow needle while keeping the guidewire in place, being careful not to allow the wire to withdraw as the needle is removed.
- Insert the catheter over the guidewire.
- Exchange the guidewire that came in the micropuncture kit with desired guidewire to begin the procedure.
Please see this video animation of the Seldinger technique for vascular access.
Closure and Closure Devices
Once the procedure is complete, percutaneous access will need to be closed. If relatively small catheters and sheaths were used during the procedure, generally below 6 French (1F = .33 millimeter), arterial access may be closed with manual compression by applying pressure with two fingers over the site of incision against the femoral head. Alternatively, certain indications, such as use of anticoagulation or large body habitus, may require closure devices even if larger catheters or sheaths were not used during the procedure.
There are several different types of closure devices. Each type can be differentiated by the use of suture or sealant to close the vessel.
A common closure device you are likely to encounter on a vascular surgery service is the Perclose ProGlide (Abbott Vascular, Chicago, IL), a suture-mediated closure device. Other closure devices include the Angioseal (Terumo, Tokyo, Japan), which deploys a bioabsorbable anchor to the arterial puncture site with surrounding collagen to promote closure, and the MYNXGRIP (Cordis, Miami Lakes, FL), which uses a balloon pressed against the site of arterial puncture and an adhesive sealant outside the vessel that dissolves slowly as the puncture site heals. Less commonly used closure devices include the Angioseal (Terumo, Tokyo, Japan), which deploys a bioabsorbable anchor to the arterial puncture site with surrounding collagen to promote closure. Other less commonly used closure devices include the Manta, StarClose, Vascade, and Cardiva.
A step-by-step guide to deploying Perclose devices is shown in this animated video and listed below.
- Exchange the procedural sheath for a guide wire so only the guide wire is left in the vessel.
- Advance the flushed perclose device over the guidewire until the wire exit port is at skin level.
- Remove the guidewire so only the perclose device is in the vessel and advance the perclose device until blood flows out of the marker lumen.
- Lift the lever to open the foot of the device.
- Insert the plunger down to insert needles, which will connect the threads of the perclose .
- Remove the plunger, which pulls the thread taught, and cut the excess thread
Lower the lever to close the foot and retract the device until the guide wire access port is visible. - Reinsert guidewire and pull both threads taught.
- Use the Snared Knot Pusher or Prostyle Suture Trimmer to advance the knot to the level of skin. 9 Pull the thread taught while removing perclose device.
- Advance the knot over the guidewire and remove the guide wire once the bleeding is controlled.
- Pull both threads tightly and remove the knot advancing device.
Wires, Catheters, and Sheaths
Using the analogy of a train on a track passing through a tunnel, the catheter can be thought of as the train, the wire as the train track, and the sheath as the surrounding tunnel.
Wires
Wires are the first device inserted into a patient, have the lowest profile, and are variable in properties. Vascular access wires are made of a metal core and an outer wire wrapped around the metal core. They are used to guide devices such as catheters and sheaths into a vessel. Characteristics of wires include:
Diameter
- Wire diameter is described in thousandths of an inch and typically range from 0.010-0.038”.
- Vascular access is typically obtained with a 0.018” wire and is exchanged for a smaller or larger profile as needed.
- The most common sizes used are 0.035”, 0.018” and 0.014”.
Stiffness
- Wire stiffness is determined by the metal used as the core of the wire.
- The same wire will become stiffer with a larger diameter.
- Softer wires are used to manipulate catheters into vessels.
- Stiffer wires are needed to push larger devices, such as balloons or endografts.
Coating
- Wires have a hydrophilic or hydrophobic coating.
- Hydrophilic wires have less friction when moving along the vessel wall and track more closely along the vessel lumen, increasing the risk of dissection.
- Hydrophilic wires are preferable in highly tortuous vessels.
- In contrast, hydrophobic wires keep more distance from the vessel wall and are less likely to dissect.
Tip Shape
- The tip shape and consistency of the wire is important.
- Angled tips are used to navigate into side branches of vessels.
- Soft tips are designed to prevent trauma to the vessel wall.
- Weighted tips are used to cross occlusions.
- J-tips are used to prevent wiring of too small of vessels that may lead to trauma of end organs.
Length
- Wires are measured in centimeters (cm) and typically range from 80 - 300 cm.
- Wires must not only be long enough to reach the target vessel from the point of access, but also need to be long enough to support exchanges outside of the body as needed.
- Care must be taken to avoid too long of a wire for its intended purpose as it is harder to control the end of the wire outside of the body.
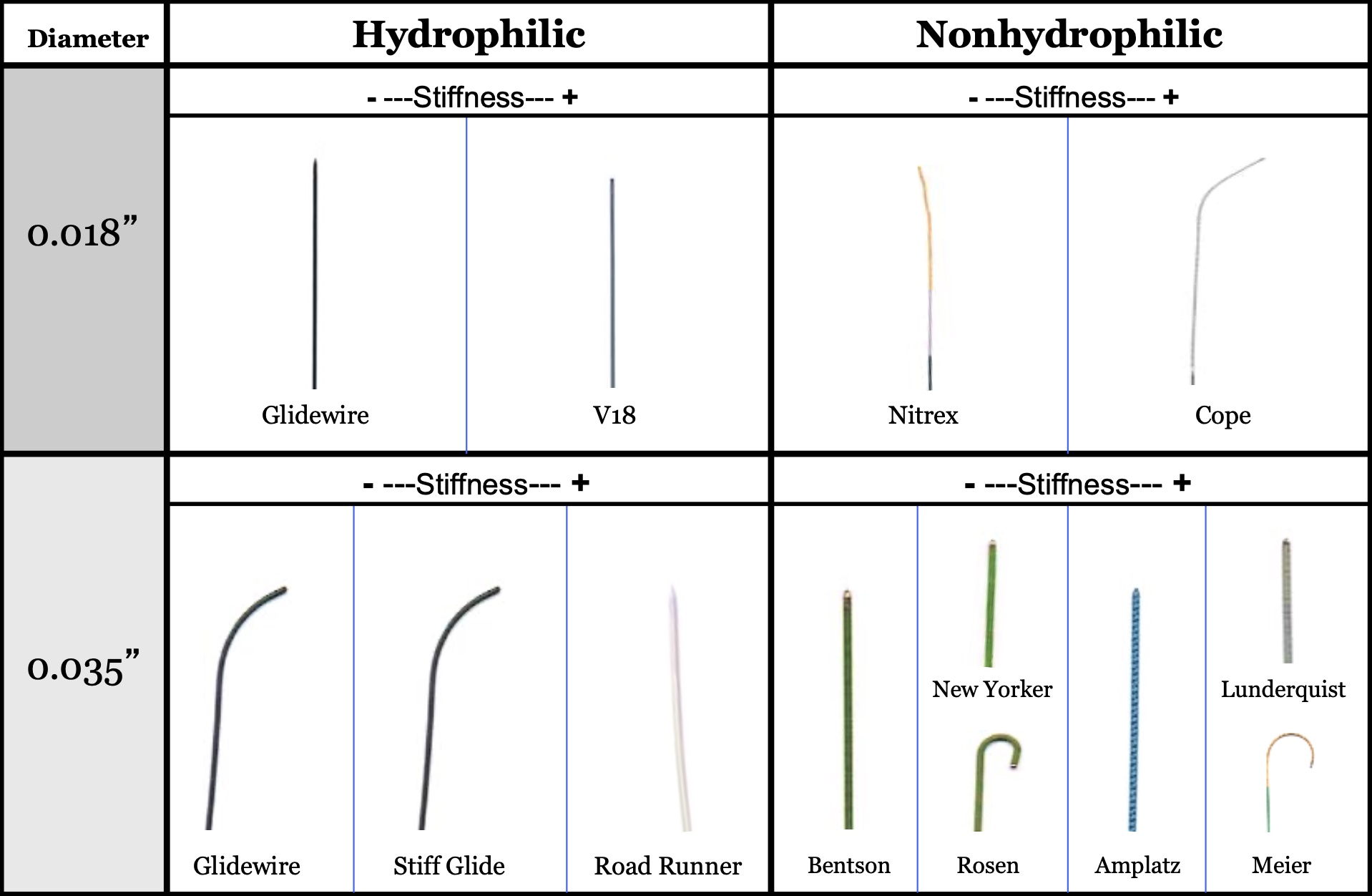
Function
Wires can be categorized by function. Please find a list of each category with common examples of specific wires below.
Access wires:
- Short, typically 0.018” wires with a soft, non-directional tip to prevent any trauma to the vessel when entering, and a stiffer body to allow tracking of the introducer and sheath into the vessel.
Guidewires:
- Generally are characterized as pushable, hydrophilic wires with a floppy, curved or angled tips that are designed to track through tortuous vessels without causing blunt trauma to the vessel wall.
- Guidewires are also used for their directionality to cannulate branches.
- Standard Glidewire (Terumo, Tokyo, Japan): can be used as an access or guidewire. Hydrophilic coating, straight or angled tip, sized 0.018” - 0.035” in diameter and 150 cm or 260 cm in length.
- Bentson (Cook Medical, Bloomington, IN): flexible wire with a hydrophobic coating and curved tip. Used to access vessels that have a slight curve, or to introduce catheters or sheaths. Comes in 0.035” diameter and 150 cm or 260 cm in length.
- Rosen (Cook medical, Bloomington, IN): stiff hydrophobic wire with J tip. Often used as the first wire exchanged for the access wire. 0.035” long and 80 cm to 260 cm in length.
Rail wires:
- Used as a platform to support catheter and sheath exchanges, as well as to advance devices, such as stents or endografts.
- Rail wires come in a range of stiffness, from medium to very stiff.
- They may have a straight, floppy, or curved tip.
Medium stiffness rail wires:
- Bentson (Cook Medical, Bloomington, IN): flexible wire with a hydrophobic coating and curved tip. Used to access vessels that have a slight curve, or to introduce catheters or sheaths. Comes in 0.035” diameter and 150 cm or 260 cm in length.
- Rosen (Cook medical, Bloomington, IN): stiff hydrophobic wire with J tip. Often used as the first wire exchanged for the access wire. 0.035” long and 80 cm to 260 cm in length.
High stiffness rail wires:
- Amplatz (Boston Scientific, Marlborough, MA): stiff, hydrophobic, straight floppy or J-tip. 0.035” - 0.038” diameter and 75 cm - 260 cm in length.
- Lunderquist (Cook Medical, Bloomington, IN): one of the stiffest wires, hydrophobic, with floppy or curved tip. 0.035” diameter and 90 cm - 300 cm in length. Often used to advance large endografts.
Catheters
Catheters are flexible, hollow tubes that are used in both the diagnosis and treatment of vascular disease.
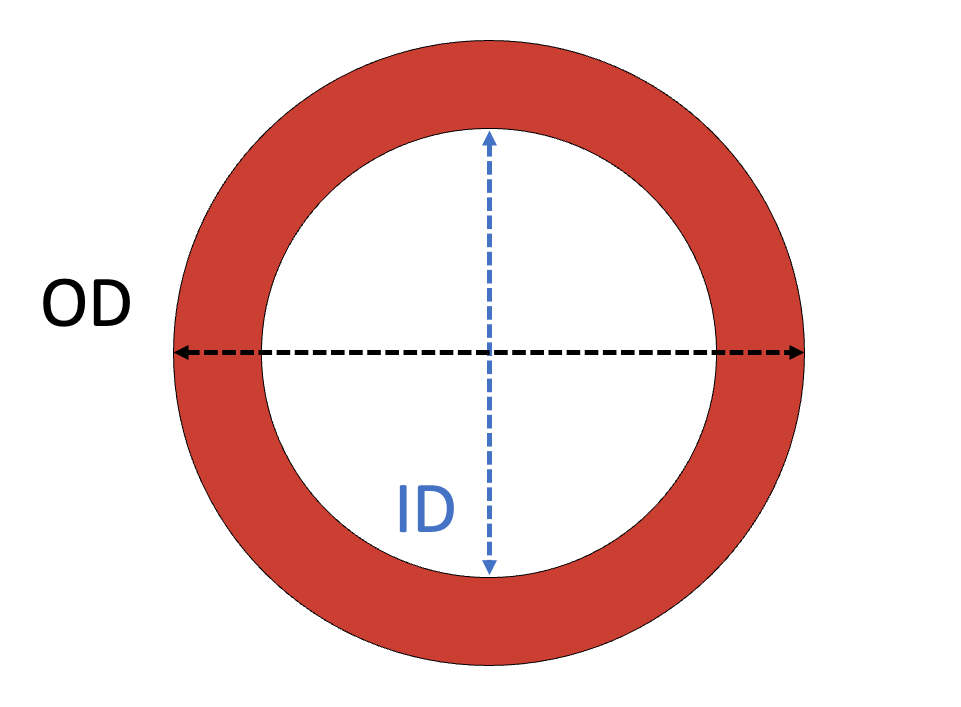
Catheters are measured in French (1F = 0.33 mm) and the diameter corresponds to the outer diameter of the tube.
Catheters vary by size, stiffness, hydrophilicity, radiopacity, and functionality, but can generally be described in two categories, flush catheters and selective catheters.
Flush catheters:
- Contain multiple side holes just proximal to the end of the device to allow for the injection of contrast or other material, such as thrombolytics.
- Omniflush (Angiodynamics, Latham, NY): soft, flexible catheter with a radiopaque tip that is able to administer contrast to visualize vessels. Omniflush device on back table
- Pigtail (Angiodynamics, Latham, NY): Similar to the Omniflush with a looped end (Image YY).
Selective catheters:
- Contain one hole at the distal end of the tube.
- Selective catheters come in many different shapes and sizes.
- Intuitively, the anatomy of the vessel to be tracked will determine the diameter and tip shape.
- When attempting to cross the aortic bifurcation into the contralateral common iliac artery, a catheter with more of a J-tip or “Shepherd’s hook” is preferable.
- When attempting to cannulate vessel branches with a more acute angle, a smaller angle catheter tip should be selected.
- Selective catheters can also be used to deliver contrast, but the contrast is only able to leave the catheter through the end of the device.
- Angiograms with selective catheters are more tailored to specific vessels, such as a renal or mesenteric artery.
- Selective Glidecath (Terumo, Tokyo, Japan): hydrophilic catheter available in 17 different shapes, but the 45 degree is most common. Comes in 4F or 5F. Offers great trackability and pushability. This image depicts some of the glidecath tip shapes.
Sheaths
Sheaths are hollow tubes that provide a conduit between outer environment (i.e. outside the body) and the vessel. Returning to our train analogy, a sheath is the “tunnel” that the track and train run through.
Sheaths are used to advance and exchange catheters, wires, stents, balloons, and other devices. Sheaths can be divided into short sheaths for access and long sheaths for diagnostics and treatment. Long sheaths are flexible enough to track along different vessels, but are also stiff enough to support the advancement or exchange of devices.
Sheaths are measured in French (1F = 0.33 mm) and the diameter corresponds to the inner diameter of the tube.
As such, a 6F catheter will pass through a 6F sheath.
Key points about sheaths:
- Sheaths are measured based on the inner diameter of the sheath, in contrast to catheters, which are measured based on their outer diameter.
- Sheaths contain a removable introducer, which is used to expand the tract from the outside into the vessel, and provide a smooth transition between the wire and the sheath.
- The side port on sheaths is used to flush the inner device with heparinized saline to prevent thrombus formation and distal embolization. It can also be used to inject contrast for diagnostic purposes.
- Sheaths come in variable diameters, lengths, and tips.
- Some sheaths, such as the TourGuide (Medtronic, Minneapolis, MN) are steerable. The operator can deflect the sheath tip up to 180 degrees. This is advantageous when cannulating branch arteries with sharp angles.
It is also helpful to understand what diameter of sheath can be inserted into different points of access as this will change the approach for the procedure. Though there is variation across patients, the table below is a useful starting point to understand what size sheath can be inserted in target access arteries.
| Artery | Inner Diameter (mm) | Typical Access Sheath size (F) |
|---|---|---|
| Radial | 2.3 | 4-6 |
| Brachial | 5-7 | |
| Axillary | 5-14 | |
| Common Femoral | 6.6 | 5-24 |
| Popliteal | 4.7 | 4-6 |
| Pedal | 2.1 | 4 |
Balloons, Stents, and Endografts
The delivery system for balloons consists of a catheter over the wire with the balloon built into the catheter tip. As such, advancing the balloon into place for angioplasty is no different from advancing a small catheter without a balloon.
Similar to balloons, stents are contained in catheters that are advanced into position over wires. The interventional component (i.e. stent) is near the tip of the catheter.
Both stents and balloons can elute drugs, most commonly Paclitaxel, an antiproliferative agent. Paclitaxel is lipophilic and remains active in the vessel wall following the procedure, preventing neointimal hyperplasia and improving patency.The impetus for using a drug-eluting stent or balloon in place of a standard stent or balloon is to decrease the chances of vascular remodeling following the procedure that may lead to restenosis.
In contrast to drug-eluting stents, drug-eluting balloons have the advantage of not leaving hardware in the vessel after intervention. Contrarily, drug-eluting stents remain in the vessel lumen after intervention, but are able to continue providing radial force.
Balloons
Balloons are versatile endovascular tools and can be categorized by the degree to which they assume of the shape of the vessel.
Compliant balloons: - Compliant balloons inflate to a specific volume and conform to the shape of the vessel. - For example, the Fogarty balloon is a compliant balloon that can be used to remove thrombi in both open and endovascular procedures.
Semi-Compliant balloons: - These balloons have a slightly more rigid shape and therefore conform to a lesser degree to the shape of the container (i.e. vessel). - The Coda balloon (Cook Medical, Bloomington, IN) is semi-compliant and used to temporarily occlude large vessels.
Non-Compliant balloons: - Non-compliant inflate to a predetermined shape and size. - They are commonly used in angioplasty of peripheral and/or visceral stenosed vessels.
Intraoperatively surgeons will often ask for the pressures of the balloon before using. Non-compliant balloons have a nominal pressure, which is the average pressure in atmospheres (atm) required to inflate the balloon to its predetermined size, and a burst pressure, where 99.9% of balloons will not burst if inflated to this pressure.
Stents
Stents may be covered or uncovered. Covered stents do not allow flow between the lumen of the stent and surrounding tissues, in contrast to uncovered (bare metal) stents which do allow flow through the stent.
Stents can be categorized by the means of deployment.
Self-expanding stents use a recoil mechanism during deployment to achieve a set diameter using stored energy from the packing of the stent.
Key characteristics of self-expanding stents:
- Flexible
- Low profile
- Deformable
- Low radial force (lower dissection risk)
Balloon-expandable stents are packaged around a balloon. The surgeon uses the balloon inside the stent to achieve a specific diameter based on the pressure used to inflate the balloon. As the diameter of the stent increases with higher balloon pressure, the length of stent decreases.
Key characteristics of balloon-expandable stents:
- Rigid
- Large profile
- Maintains shape over time
- More accurate deployment
- High radial force (higher risk of dissection)
Highly tortuous vessels often require a self-expanding stent because of the increased flexibility needed to prevent kinking or in-stent restenosis.
Heavily calcified vessels often require the increased radial force of balloon-expandable stents to achieve full patency.
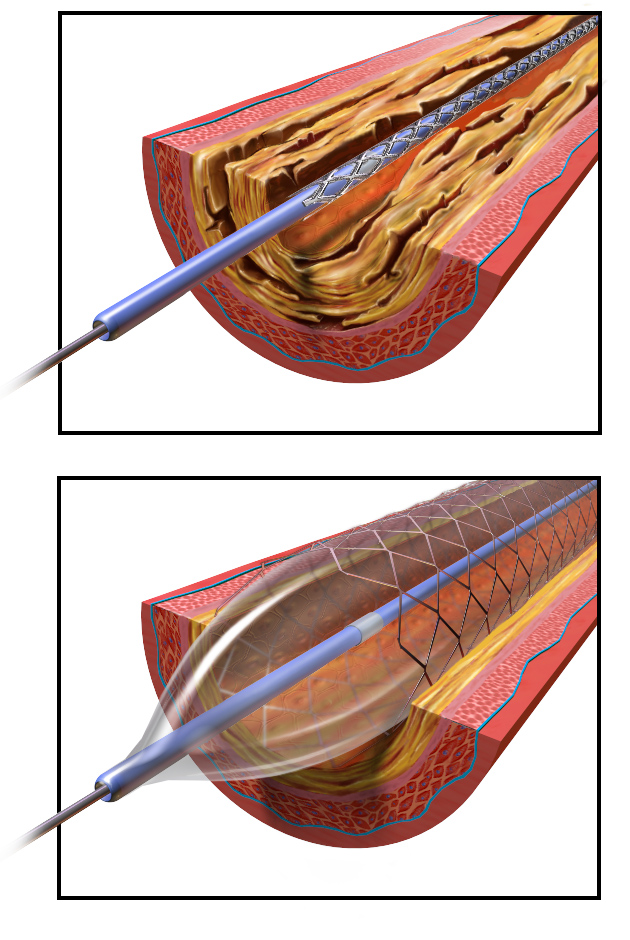
Endografts
Endografts are tubes of fabric and interwoven metal used to exclude the diseased segment of an artery from circulation. The goal of an endograft is to prevent rupture or sac enlargement of an aneurysm by depressurizing the arterial walls.
Endografts are comparatively larger than stents and can require very large sheaths (>18F for endovascular aortic repair). To treat an aortic aneurysm with an endograft, there must be adequate sealing zones of healthy aorta above and below the aneurysm.
Please see the AAA Chapter for a full review of endovascular aortic repair (EVAR).
Please see the TAA/TAAA Chapter for a full review of thoracic endovascualr aortic repair (TEVAR)
For patients that have extension of the aneurysm into the visceral vessels of the aorta, the use of a conventional, non-fenestrated endograft would block blood flow to the branches of the diseased segment of the aorta, such as the kidneys or bowel. Treatment of thoracoabdominal or complex abdominal aortic aneurysms with endovascular repair requires a graft that allows flow to branches of the aorta. Some common techniques for maintaining visceral blood flow include :
Fenestrated endografts include small, circular holes in the main body of the endograft. The french word for window is “fenêtre.” When the fenstrations (or windows) are placed in line with visceral vessel origins, balloon-expandable stents can be used to bridge to the main endograft to the native renal or mesenteric arteries.
Additional characteristics about fenestrated grafts:
- They are ideal when the inner aortic diameter is small, making for a short distance (<5 mm) between the endograft and native visceral artery.
- They require the pathway between the endograft and the native artery to be close to perpendicular; that is, the pathway for the stent out of the graft cannot be at too large of an angle due to risk of stent fracture or kinking.
- The part of the stent inside the endograft must be flared out with a balloon to prevent endoleak from inside the endograft into the aneurysm sac.
- An of a commercially available fenestrated endograft is the Cook Medical Zenith Fenestrated (ZFen) AAA endograft. Click here to watch an animation of ZFen deployment.
Branched endografts have side branches built-into the main body of the graft in place of fenestration. These branches extend from the outer body of the main graft towards the target vessel.
Additional characteristics about branched grafts:
- They require a larger inner aortic diameter for more space between the endograft and target artery.
- Branches can receive self-expanding or balloon-expandable stents.
Common Endovascular Procedures
Vascular surgeons perform a number of endovascular procedures. The most common include placement of an inferior vena cava (IVC) filter, diagnostic angiograms with angioplasty and stenting, fistulagrams, endovascular aneurysm repair (EVAR), and thrombectomy and thrombolysis. There are a number of basics that trainees should know before starting one of these procedures.
IVC Filter Placement
- An IVC Filter is a metal cage-like device placed in the IVC to prevent clots from embolizing to the pulmonary vasculature.
- The hook at the superior aspect of the filter is used to aid with retrieval.
- The struts will catch clots and will anchor into the walls of the IVC to prevent migration.
- Placement of an IVC filter can be controversial but it is commonly used to prevent pulmonary embolism in patients who have a strict contraindication to anticoagulation.
- They are sometimes placed prophylactically in high-risk trauma patients.
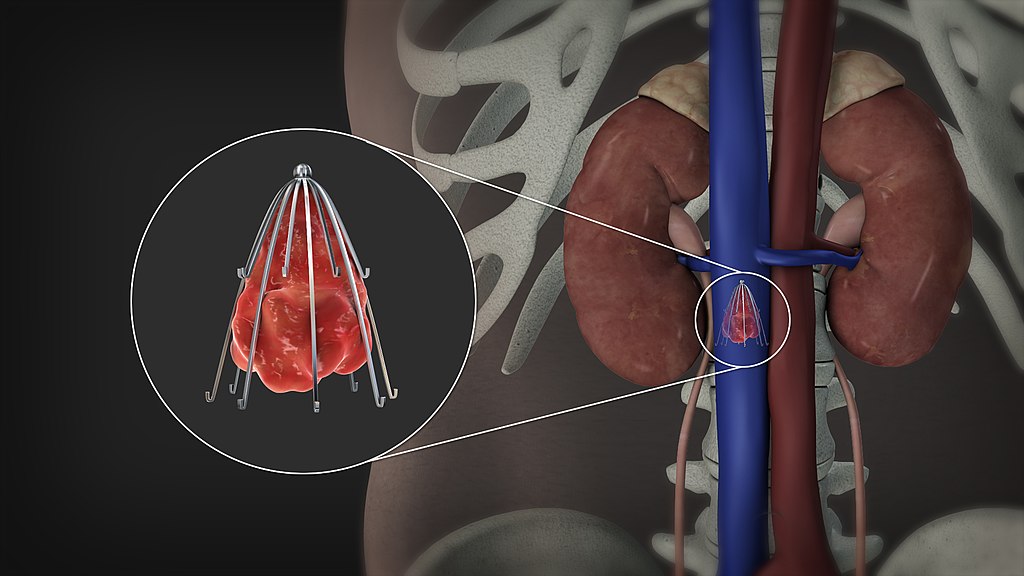
Procedure:
Access is usually obtained via the right femoral vein as it gives direct access to the IVC.
Ultrasound should be used to confirm that there is no thrombus at the access site - Access is then obtained via the Seldinger technique and a venacavogram is then obtained to assess the anatomy and the location of the renal veins
The filter is generally placed just below the renal veins to prevent renal vein thrombus if filter thrombosis occurs.
If a retrievable filter is placed, it should be removed as soon as the filter is no longer clinically indicated.
Filter retrieval becomes more challenging the longer the filter remains in place.
Filter retrieval is generally performed through the internal jugular vein in the neck to give access to the hook at the superior end of the filter, which can be snared and removed.
Please find a more detailed review of IVC filters here
Lower Extremity Angiogram
A lower extremity angiogram is the starting point for a vascular surgeon to perform a number of different interventions including angioplasty, stenting, and thrombectomy. An angiogram is a procedure in which contrast dye is used to display the vasculature and its disease burden under X-ray.
Before an angiogram, one should be aware of all prior interventions a patient has had, both open and endovascular. It can be useful to review old operative reports and imaging studies in order to know the patient’s current anatomy.
In addition, the patient’s most recent imaging studies should be reviewed including ABI/PVRs, arterial duplex, and any axial imaging such as a CTA.
Please see Claudication Chapter for a summary of ABI/PVR testing and interpretation.
Procedure:
Access is typically obtained in the contralateral common femoral artery and a wire and catheter are passed up and over the aortic bifurcation to gain access to the operative (ipsilateral) side.
A series of angiograms are taken to assess the level of disease and based on this a final operative plan will be made.
There are multiple options for treatment including angioplasty, stenting, atherectomy, thrombectomy, and thrombolysis, to name a few.
Possible Interventions:
Angioplasty: a balloon is advanced across the area of concern (usually atherosclerosis causing a flow limitation) and expanded to dilate the area of stenosis.
Stenting: commonly after an angioplasty, a stent is placed to maintain patency of the area. There are multiple types of stents (covered above) that can be used depending on anatomic location, size, and indication.
Atherectomy: a procedure where a calcified plaque is physically removed from the artery. This can be done with a cutting blade, a spinning drill-like device, or a laser.
Considerations for Atherectomy
As plaque is broken into smaller pieces, there is a significant risk for distal embolization of plaque debris. To prevent this, an embolic protection device is often used which can filter blood and catch any smaller particulate matter that travels distally. Some of the devices include an aspiration device to remove clot and thrombus as it is removed.
There is ongoing research into the outcomes of atherectomy versus stenting alone, but generally, the long-term patency rate is similar.(“Atherectomy for Lower Extremity Intervention: Why, When, and Which Device?” n.d.)
Thrombolysis: a procedure where the clot is dissolved often with tPA and heparin to treat an acute thrombotic event. Often, angiograms are performed for acute limb ischemia from a clot rather than atherosclerotic disease. A lysis catheter or Cragg-Macnamara catheter can be placed. The Cragg-Macnamara catheter is a long catheter with multiple side ports where medication can be infused over the length of a thrombosed artery. This catheter can be left in place for 24-48 hours and the medication is allowed to “bust” the acute clot. After this, the patient will be brought back to the operating room to evaluate the progress of the clot dissolution.(Giannakakis et al. 2017)
Thrombectomy: a procedure where acute or subacute thrombus is removed from a vessel. This can be accomplished in a variety of ways. Commonly, a suction thrombectomy system can be used to remove thrombus from a vessel using vacuum force for example using the Penumbra system. Alternatively, a mechanical thrombectomy can be performed where a Fogarty catheter is deployed past a thrombus, a balloon inflated at the end of the catheter, and then manually pulled back towards an arteriotomy and the clot then removed.(Karnabatidis et al. 2011)
Fistulograms
A fistulogram is an angiographic study used to interrogate and troubleshoot a disfunctional arteriovenous fistula.
Common complications of fistulas include:
- Anastomotic strictures.
- Inflow issues (such as atherosclerosis of the inflow artery).
- Outflow issues (such as central venous stenosis).
- Thrombosed fistula.
Access is obtained in different locations depending on the culprit lesions (the inflow artery, the fistula itself, the outflow veins) and can be treated with angioplasty and or stenting.
Risks
As with any invasive procedure, even a minimally invasive procedure comes with its own set of risks, and as the complexity of the procedure increases so does the risk.
The American Heart Association has put out guidelines to help stratify patients at risk for vascular complications.(Patel et al. 2010a)
Low risk cases were defined as those with a complication rate below 1%. Characteristics include:
- Diagnostic angiograms without interventions.
- Short operative times.
- Small sheaths (5 Fr).
- Minimal anticoagulation.
- Younger male patients with larger body habitus and normal renal function were at lowest risk of complications.
Moderate risk cases were defined as those with a complication rate of 1-3%. Risk factors include:
- Longer, more complex procedures.
- Involve larger sheaths (6-7Fr).
High risk cases were defined as those with a complication rate >3%. Risk factors include:
- Peripheral arterial disease patients
- Advanced age
- Female sex at birth
- Liver disease
- Coagulopathy
- Immunosuppression
- Status post valve replacement
- Renal dysfunction.
- Longer procedure times
- Larger sheaths (>8Fr), use of arterial closure devices
- More likely to involve anticoagulation
Access Complications
Although each procedure comes with its own specific risks (e.g. stroke for a carotid artery stent, distal embolization for atherectomy etc), there are multiple complications that can occur with any endovascular procedure. The procedure specific complications will be explored in further detail in each chapter. Common access complications are listed below.
Groin Hematoma
A groin hematoma is a collection of blood that has collected outside the lumen of the vessel, which often occurs after removal of the sheath.
Groin hematoma characteristics:
- Occur in about 1-3 % of cases
- Often due to a stick not directly over the femoral head in which case manual pressure may not provide adequate hemostasis.
- Hematomas are rarely ever operated on unless they case skin compromise, severe pain, femoral nerve compression, or are rapidly expanding.
- Hematoma will generally be absorbed by the body.
- If they are operated on, there is a significant risk of infection (about 20%) and seroma formation.
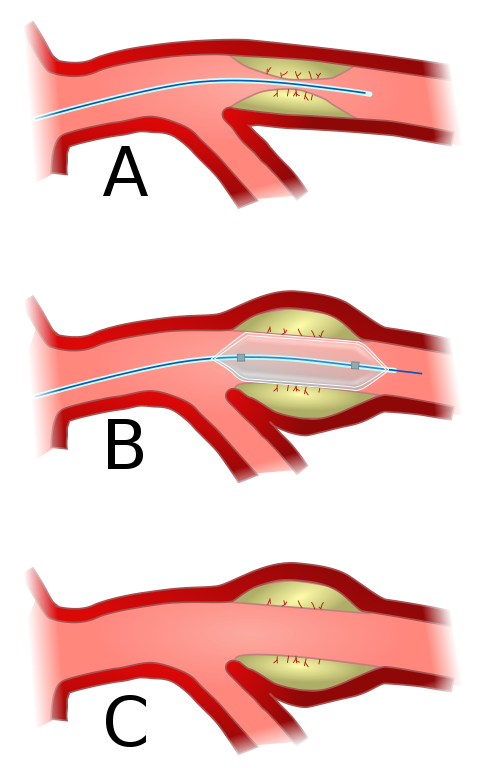
Pseudoaneurysms
A pseudoaneurysm is defined as a collection of turbulent blood flow contained only by one or two layers of the arterial wall as opposed to a true aneurysm where all three layers are dilated (occur in less than 1% of endovascular cases). They occur secondary to injury (iatrogenic or trauma).
These must be managed urgently and treated appropriately to prevent significant morbidity and mortality.
Pseudoaneurysm presentation:
- Pulsatile often painful mass at a prior access site
- May lead to skin necrosis
- Bruit on auscultation
Pseudoaneurysm risks:
- Vessel rupture
- Thrombus formation with distal embolization
- Skin necrosis
- Nerve compression
- Venous compression
Etiology:
Most often, seen at the femoral artery as an access site complication from:
- Failed deployment of a closure device
- Laceration of the artery or branch by an access needle
- Inadequate pressure or length of pressure being held.
Patients who are obese, female, hypertensive or are on anticoagulation or antiplatelet therapy are at higher risk for femoral PSA.
Workup:
- Duplex ultrasound of the site.
- Pulse exam to ensure adequate distal perfusion.
Management:
Management is dependent on size and presentation of the pseudoaneurysm. In the past, all pseudoaneurysms were treated operatively, but with new advances, minimally invasive techniques have become more common with good outcomes.
- For a small asymptomatic pseudoaneurysm (less than 2cm), an appropriate first step would be observation and repeat duplex ultrasound in about one month.
- For a persistent pseudoaneurysm after one month, a larger/loculated asymptomatic pseudoaneurysm, or a pseudoaneurysm that presents with minor pain at the site, minimally invasive intervention with ultrasound guided compression or ultrasound guided thrombin injection is appropriate.
- For any pseudoaneurysm presenting with persistent hemodynamic instability, limb ischemia, skin necrosis, AV fistula, nerve compression, expanding hematoma, an urgent operation is required.
- Urgent open repair generally consists of gaining proximal and distal control and then primary repair of the arterial defect.
- Urgent endovascular management may also be entailed by deploying coils in the sac and placing a covered stent in the femoral artery, therefore thrombosing the pseudoaneurysm.
[Kassem et al. (2013)](Kassem and Elmahdy 2014)(Tulla, Kowalski, and Qaja 2024)
Retroperitoneal Hematoma
A retroperitoneal hematoma is a collection of blood accumulating in the retroperitoneal space where the external iliac artery travels.
Key points:
- It is a rare complication occurring in about 0.5% of cases.
- Retroperitonaly hematomas are a dreaded complication as they comes with high morbidity and mortality especially if not rapidly diagnosed.
- Retroperitoneal hematomas occur when the arterial stick in the artery during groin puncture is performed too high, above the level of the inferior epigastric artery.
- This leads to extravasation of blood into the retroperitoneal space after sheath removal.
- Retroperitoneal hematomas can usually can be treated with a closure device if diagnosed intra operatively or a covered stent from the contralateral side.
Arteriovenous (AV) Fistula
An arteriovenous fistula is an aberrant connection between an artery and a vein. Although commonly formed surgically for hemodialysis access, they can be caused iatrogenically during arterial access for endovascular intervention.
Key points:
- They are rare and occur in less than 1% of groin access cases.
- Occur during inadvertent puncture of the deep femoral artery and deep femoral vein.
- Usually no intervention is needed.
Dissection
An arterial dissection is the separation of the layers of the arterial wall.
Key points:
- May occur naturally (often secondary to hypertension) or iatrogenically (in the setting of arterial puncture). This most often occurs in the setting of a posteriorly lying plaque in the artery where the wire can separate the posterior layers.
- To prevent dissection, do not apply too much forward pressure when advancing a wire during initial arterial access. Do not inject fluid (saline or contrast) unless there is blood return to ensure the needle or catheter tip are in the true lumen.
- Treatment can be conservative in the case of an iatrogenic dissection or treated with stent placement if the dissection limits blood flow. Intravascular ultrasound can be used to further assess the dissection.
Acute Thrombosis of Access Vessel
Acute thrombus of the access vessel is when the artery where access is obtained completely “clots off.” It is an incredibly rare complication caused by incorrect deployment of vascular closure devices, access site dissection, or occlusive sheath placement in a small or diseased vessel.
Endovascular Surgical Complications
Atheroembolism
Atheroembolism is the dislodgement of a portion of a cholesterol plaque leading to distal embolization causing acute thrombosis or partial occlusion.
Key points: (Liew and Bartholomew 2005)
- Generally, when these come from the ascending aorta or arch, they will embolize to the cerebral and retinal vessels. On the other hand, when an embolus originates in the thoracic or descending aorta, it will likely embolize to the mesenteric, renal or lower extremity vessels.
- These emboli will then cause a further inflammatory response worsening outcomes.
- Outcomes are dependent on volume and size of emboli and can range from sub-clinical to fatal.
- Embolization is most common to the skin, kidneys, and GI tract though it is dependent on the site of the procedure.
- Unfortunately this condition is generally under diagnosed and challenging to treat. - Primary prevention is of utmost importance with good surgical technique.
Wire Perforation
Wire perforation occurs when an angiographic wire creates a hole in a vessel.
Key points:
- Incredibly rare but treatable when diagnosed rapidly.
- Occurs when a large stiff hydrophilic wire is passed through a small vessel especially if not done under fluoroscopic guidance.
- Treatment involves transcatheter embolization of the perforation site.
Vessel Rupture
Vessel rupture occurs when a device or balloon is oversized for a vessel and causes a “blowout” of a vessel.
Key points:
- Rupture can be cause by an oversized balloon or balloon expandable stent, by expanding a properly sized balloon in a heavily calcified vessel or by passage of a large device such as an EVAR device through a small vessel.
- Rupture is associated with a high morbidity and mortality especially if there is any delay in diagnosis.
- Immediately upon recognition of vessel rupture, a balloon should be inflated in the region to obtain adequate hemostasis. Simultaneously, supplies should be can gathered for vessel repair.
- Repair is usually done with a covered stent placed across the rupture. If adequate hemostasis cannot be obtained, open repair may be necessary.
Teaching Case
Scenario
A 71 year old male with a past medical history of hypertension, hyperlipidemia, coronary artery disease with stent placement was diagnosed with acute type B aortic dissection three weeks ago. He was managed medically in the hospital and sent home after one week with blood pressure and pain well-controlled. An image from the CTA at time of diagnosis is seen below:
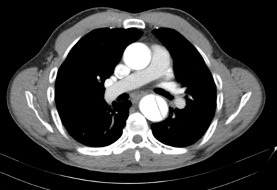
The proximal extent of the dissection was 3 cm distal to the left subclavian artery and the distal extent was 2 cm superior to the celiac artery, allowing good landing zones for an endograft. The left internal iliac artery is fully occluded, and the right internal iliac artery has roughly 50% stenosis. The left external iliac artery is heavily calcified and stenotic >50%. The right common and external iliac artery are patent without evidence of significant atherosclerosis. The patient is evaluated in the preoperative area prior to placement of a thoracic endograft in the hybrid operating room.
Exam
HEENT: Pupils round and reactive to light, no lymphadenopathy
Cardiac: Regular rate and rhythm
Pulmonary: Clear to auscultation in all lung fields. No accessory muscle use.
Abdominal: Soft, non-tender, non-distended, no pulsatile masses felt.
Vascular/Extremities: Warm, non-edematous. Palpable pulses in all four extremities.
Discussion Points
However, we feel this chapter contains all the necessary information to answer the questions. If not, please let us know!
- What clinical risk factors make this patient a good candidate for endovascular vs. open repair?
- The endograft used for this operation requires at least an 18F sheath, which site of access should be used to insert and implant the graft?
- What diameter and hydrophilicity of wire should be used to advance the thoracic endograft to the site of dissection?
- The attending surgeon requested mean arterial pressures to be at least 90 mmHg throughout the operation. Why would intraoperative hypotension be concerning in this patient? What anatomic factors make this patient at particular risk?
- If the patient developed pain at the site of access one week later, with a well-healing wound and palpable thrill on physical exam, what kind of access-related complication are you worried about and what would be your next step to confirm the diagnosis?
Key Articles
Patel MR, Jneid H, Derdeyn CP, Klein LW, Levine GN, Lookstein RA, White CJ, Yeghiazarians Y, Rosenfield K; American Heart Association Diagnostic and Interventional Cardiac Catheterization Committee of the Council on Clinical Cardiology, Council on Cardiovascular Radiology and Intervention, Council on Peripheral Vascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Arteriotomy closure devices for cardiovascular procedures: a scientific statement from the American Heart Association. Circulation. 2010 Nov 2;122(18):1882-93. doi: 10.1161/CIR.0b013e3181f9b345. Epub 2010 Oct 4. Erratum in: Circulation. 2010 Nov 2;122(18):e507. PMID: 20921445. (Patel et al. 2010b)
Siracuse JJ, Farber A, Cheng TW, Raulli SJ, Jones DW, Kalish JA, Smeds MR, Rybin D, Schermerhorn ML; Vascular Quality Initiative. Common femoral artery antegrade and retrograde approaches have similar access site complications. J Vasc Surg. 2019 Apr;69(4):1160-1166.e2. doi: 10.1016/j.jvs.2018.06.226. Epub 2018 Dec 4. PMID: 30527937. (Siracuse et al. 2019)
Additional Resources
Audible Bleeding Content
- Holding Pressure Case Prep: Endovascular Basics
- Audible Bleeding Exam Prep: Endovascular Access
Websites
- TeachMe Surgery: Pseudoaneurysm
Serious Games
Touch Surgery Simulations.
- Must download the Medtronic Touch Surgery mobile application to access the modules. Available for Apple and Android mobile devices.
- Femoral Artery Access
Gore Combat Manual
The Gore Medical Vascular and Endovascular Surgery Combat Manual is an informative and entertaining read intended as a vascular surgery crash course for medical students, residents, and fellows alike. Highly accessible with a thoughtfully determined level of detail, but lacking in learning activities (e.g. questions, videos, etc.), this resource is a wonderful complement to the APDVS eBook.
Please see pages 25-41, 49-61.
Operative Footage
Developed by the Debakey Institute for Cardiovascular Education & Training at Houston Methodist. YouTube account required as video content is age-restricted. Please create and/or log in to your YouTube account to have access to the videos.